When this guest post is published, please add this guestpost’s live link to the following existing article of your site:
Internal link: https://charfen.co.uk/how-can-rapid-prototyping-help-medical-device-companies-shorten-product-development-cycles-by-60/
Anchor text: competitive medical technology
Introduction
Scientific discovery often moves faster than manufacturing capability. Many antibody candidates demonstrate strong early performance but stall when larger quantities are required. The World Health Organization has noted that production scalability remains a bottleneck in translating promising biologics into real-world solutions.
This places large scale antibody production at the heart of modern research and development, not only as a manufacturing function, but as a competitive medical technology differentiator. In an environment where multiple teams may pursue similar targets, the ability to scale reliably and early can determine which programs advance and which fall behind. Scaling antibody expression is no longer just about volume. It is about maintaining quality, consistency, functional integrity, and development velocity as demand increases.
What Is Large Scale Antibody Production?
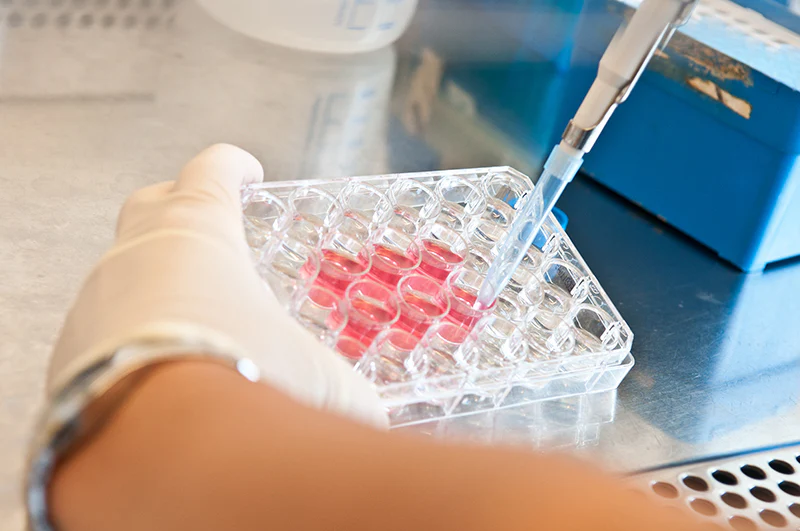
Large scale antibody production refers to the expansion of antibody expression systems, typically mammalian cells, into controlled bioreactor environments capable of generating large quantities of material with reproducible quality attributes.
This process involves upstream cell culture optimization and downstream purification, both of which must be tightly controlled. Parameters such as nutrient supply, oxygenation, shear stress, and waste removal influence antibody yield, structural integrity, and post-translational modifications.
From a competitive technology standpoint, scale introduces new variables that expose the maturity of a production platform. Processes that perform well at bench scale may behave unpredictably in larger systems. Teams that anticipate and control these effects gain a measurable advantage, particularly when timelines are compressed or comparability across batches becomes critical.
Real-World Impact of Large Scale Antibody Production
Large scale production supports preclinical efficacy studies, toxicology testing, diagnostic manufacturing, and clinical trial supply. Without scalable production, even the most promising antibodies remain confined to early research, unable to compete for clinical or commercial relevance.
Publications in Nature emphasize how subtle changes in manufacturing conditions can affect antibody structure, glycosylation patterns, and biological activity. These attributes directly influence safety, efficacy, and regulatory outcomes.
In competitive medical technology landscapes, this consistency becomes strategic. Programs that can demonstrate stable, scalable production earlier are better positioned to attract partners, progress through regulatory milestones, and respond to shifting clinical priorities. Large scale antibody production therefore acts as both a scientific enabler and a competitive filter.
Why Large Scale Antibody Production Matters for the Future
Antibody-based modalities are expanding rapidly. Bispecific antibodies, antibody-drug conjugates, and engineered formats all demand robust and adaptable production strategies that can keep pace with innovation.
As global health challenges continue to emerge, competitive advantage increasingly depends on speed and reliability. Rapid response capabilities require production pipelines that are already validated at scale, not improvised under pressure. Large scale antibody production enables this readiness by embedding scalability into development from the outset.
From a regulatory and technology perspective, scalable production also supports clearer documentation, comparability studies, and process validation. These elements reduce downstream friction and strengthen a program’s overall development position.
Benefits for Research and Development Teams
For researchers, scalable production reduces delays between discovery and application. Experiments can proceed without material shortages or disruptive batch variability.
For development teams, early attention to large scale antibody production improves decision-making. Manufacturing feasibility becomes part of candidate selection, not a late-stage risk. This integration shortens development cycles and reduces costly redesigns.
For healthcare systems and technology ecosystems, scalable antibody production supports broader access to validated antibody-based tools and therapies, reinforcing competitiveness at both institutional and national levels.
Conclusion
Discovery alone is not enough. Translation requires execution, and execution increasingly defines competitive medical technology leadership. Large scale antibody production ensures that antibodies retain their intended properties as they move from laboratory research to broader application.
As antibody pipelines grow more complex and crowded, large scale antibody production will remain essential not only for scientific success, but for sustaining competitive advantage in translational and clinical innovation.